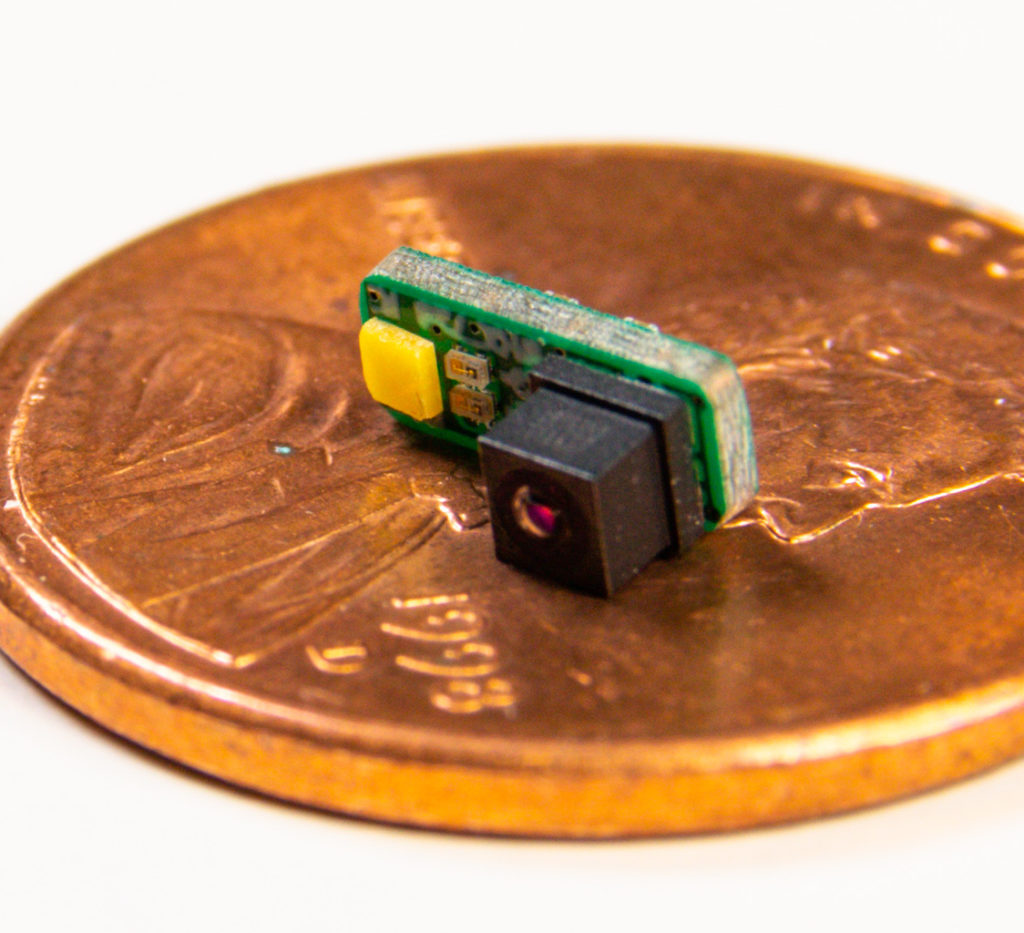
Lighthouse Imaging Optical Product Development Services
Lighthouse Imaging provides complete turn-key optical product development services and solutions for the most challenging of visualization applications. Our end-to-end optical expertise for medical visualization devices puts surgeons in the driver’s seat.
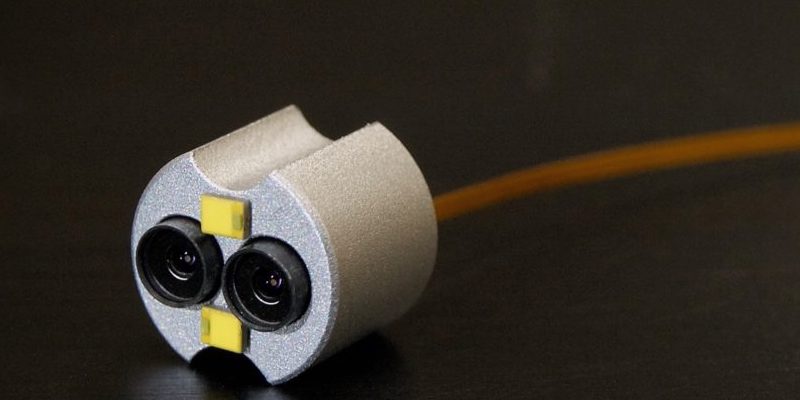
Visualization System Design
At Lighthouse Imaging, we work with our OEM partners to create solutions for their end customers that meet their demands and chalenging work environments. Visualization is the primary link between a surgeon and their work. This interface must be seamless and immersive, which is why our holistic optical design approach enables our clients to deliver medical devices of the highest caliber.
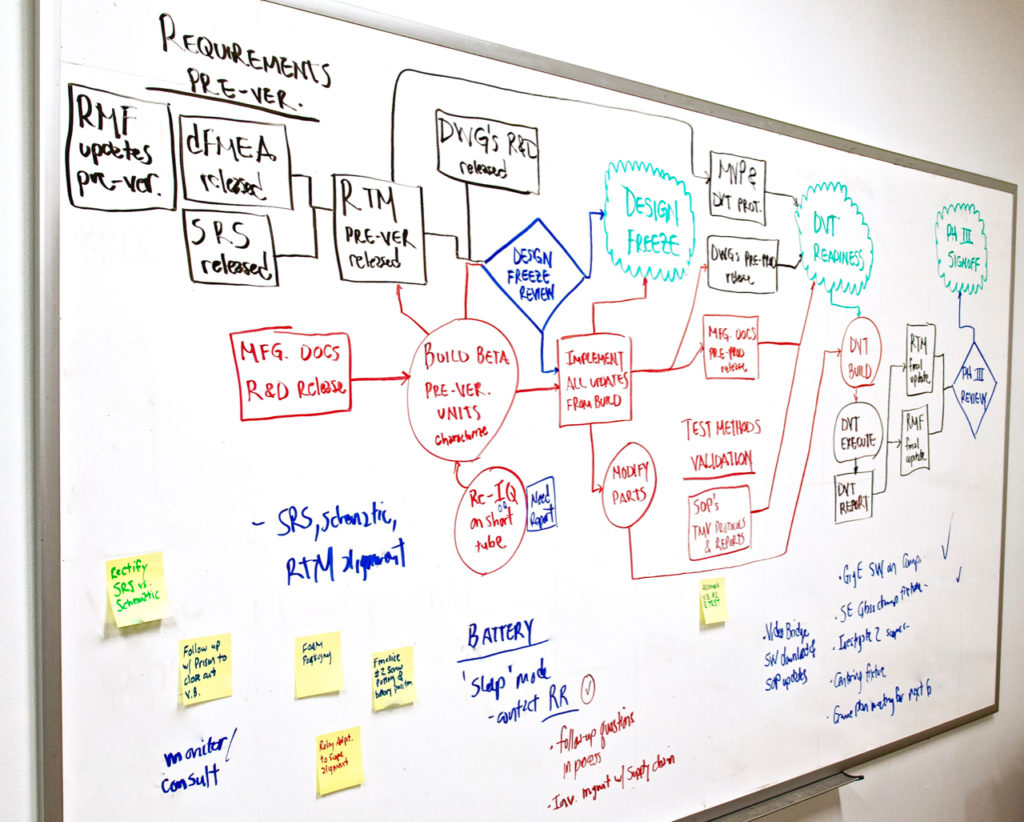
Optical Product Development Process
Lighthouse utilizes the phase/gate process that takes optical medical devices from concept through commercialization. We understand the importance of design control and risk management activities and incorporate these into our rigorous product development process.
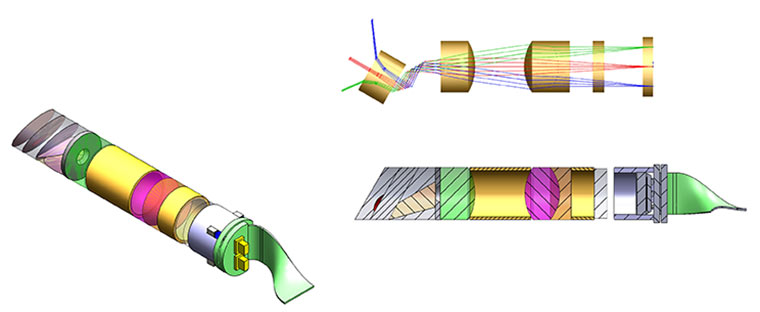
Optical Design & Optical Manufacturing Competencies
Product development competencies at Lighthouse Imaging include Systems, Optical, Mechanical, Electrical and Process Development Engineering. Our product development team has extensive experience developing visualization systems that are used in a variety of clinical applications.
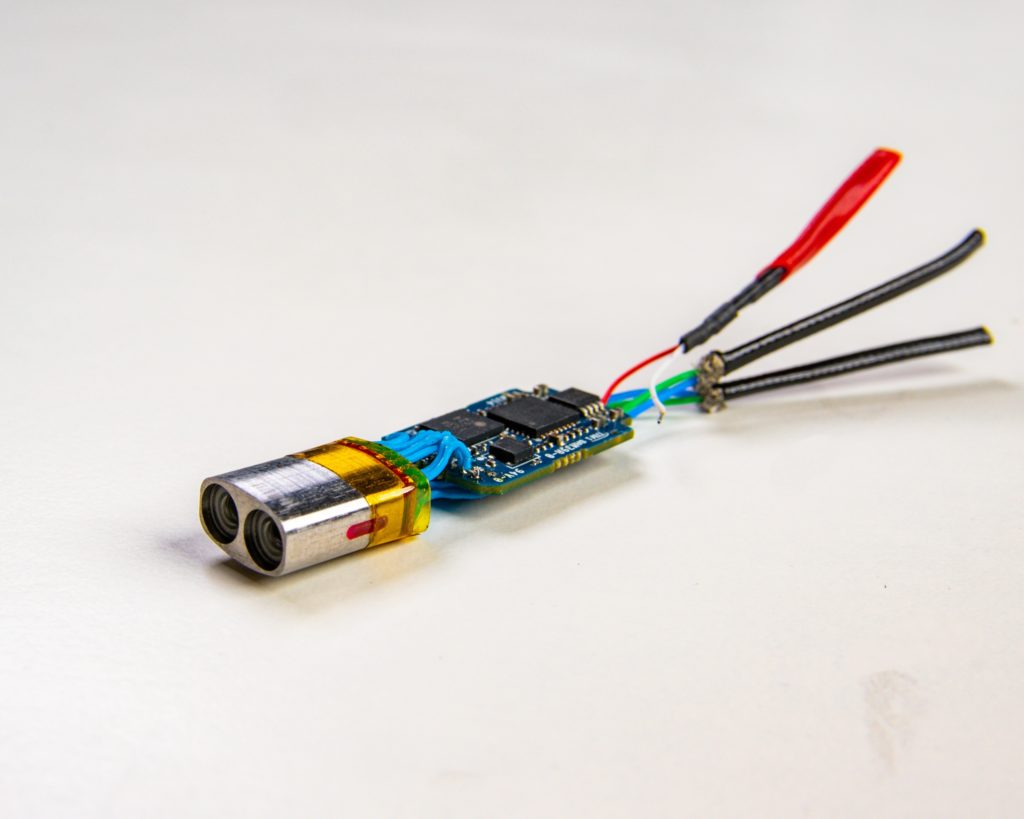
Chip-On-Tip-Design and 3D Optical System Design
Lighthouse Imaging is the leading optical development partner and contract manufacture for chip on tip visualization systems. By focusing on miniaturization and performance, we enable vision for true minimally invasive surgical procedures. Lighthouse has also pioneered the field of 3D stereoscopic endoscopy and we continue to lead the industry for custom development and manufacturing of 3D systems.

Miniature Optics for Medical Devices
Lighthouse Imaging is an acknowledged leader in miniature visualization devices and endoscopic equipment.
